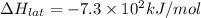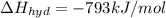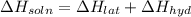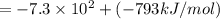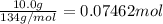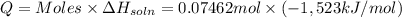Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

You know the right answer?
Lithium iodide has a lattice energy of −7.3×102kj/mol and a heat of hydration of −793kj/mol. find th...
Questions




Physics, 06.05.2020 04:44


Chemistry, 06.05.2020 04:44


Mathematics, 06.05.2020 04:44









History, 06.05.2020 04:44

Mathematics, 06.05.2020 04:44

Spanish, 06.05.2020 04:44