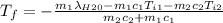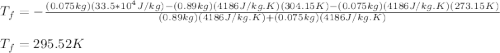Physics, 06.05.2020 04:44 Artemis3821
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it. The heat capacity of both water and tea is c = 4186 J/(kg⋅K), and the latent heat of fusion for water is Lf = 33.5 × 104 J/kg. show answer No Attempt 50% Part (a) Input an expression for the final temperature after the ice has melted and the system has reached thermal equilibrium. Part (b) What is the final temperature in Kelvin?

Answers: 2


Another question on Physics

Physics, 22.06.2019 10:00
Your town is considering building a biodiesel power plant describe at least two advantages and two disadvantages
Answers: 1

Physics, 22.06.2019 13:20
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1

Physics, 22.06.2019 14:30
Suppose that 27 j of work is needed to stretch a spring from its natural length of 6 m to a length of 9 m. (a) how much work is needed to stretch the spring from 12 m to 14 m? j (b) how far beyond its natural length will a force of 78 n keep the spring stretched?
Answers: 2
You know the right answer?
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it....
Questions

Mathematics, 13.03.2020 19:46


Physics, 13.03.2020 19:46

Mathematics, 13.03.2020 19:46





Mathematics, 13.03.2020 19:46

Social Studies, 13.03.2020 19:46


English, 13.03.2020 19:46








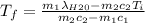
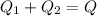
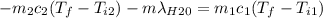 ( 1 )
( 1 )