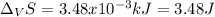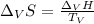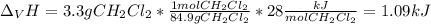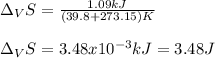Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
The heat of vaporization ΔHv of dichloromethane CH2Cl2 is 28.0 /kJmol . Calculate the change in entr...
Questions

Geography, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10



Mathematics, 08.06.2021 18:10


Mathematics, 08.06.2021 18:10

Law, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10



Arts, 08.06.2021 18:10



Biology, 08.06.2021 18:10


Spanish, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10

Social Studies, 08.06.2021 18:10