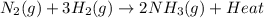Chemistry, 23.04.2020 00:08 shadowsnake
Ammonia is produced commercially by the Haber reaction: N2(8)+ 3H2(8)
The formation of ammonia is favored by
2NH3(8) + heat
an increase in pressure
a decrease in pressure
removal of N2(g)
4.
removal of H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
Ammonia is produced commercially by the Haber reaction: N2(8)+ 3H2(8)
The formation of a...
The formation of a...
Questions




Computers and Technology, 26.09.2021 14:40

Social Studies, 26.09.2021 14:40





Mathematics, 26.09.2021 14:40

Mathematics, 26.09.2021 14:40

Mathematics, 26.09.2021 14:40

History, 26.09.2021 14:40





Mathematics, 26.09.2021 14:40


Mathematics, 26.09.2021 14:40