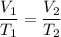Chemistry, 22.04.2020 05:28 twistedhyperboles
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The original temperature of the gas was 282K and the pressure remains constant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The or...
Questions

Social Studies, 27.09.2021 14:20


Physics, 27.09.2021 14:20

Mathematics, 27.09.2021 14:20

Biology, 27.09.2021 14:20


Chemistry, 27.09.2021 14:20



Mathematics, 27.09.2021 14:20


World Languages, 27.09.2021 14:20

Mathematics, 27.09.2021 14:20




Physics, 27.09.2021 14:20

Mathematics, 27.09.2021 14:20

Mathematics, 27.09.2021 14:20