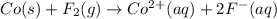Assign oxidation states to all of the species in the following redox reaction. For the reactants, identify electron loss or gain, the species oxidized, the species reduced, the oxidizing agent and the reducing agent. Co(s) + F2(g) Co2+(aq) + 2F-(aq) Oxidation state Electron loss or gain Oxidized or reduced Reducing or oxidizing agent

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Assign oxidation states to all of the species in the following redox reaction. For the reactants, id...
Questions



Social Studies, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31

Social Studies, 18.01.2020 21:31


Mathematics, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31


Mathematics, 18.01.2020 21:31

History, 18.01.2020 21:31

History, 18.01.2020 21:31

Biology, 18.01.2020 21:31

Biology, 18.01.2020 21:31



Mathematics, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31

Physics, 18.01.2020 21:31

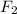 gain two electrons and thus gets reduced and acts as oxidizing agent.
gain two electrons and thus gets reduced and acts as oxidizing agent.