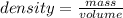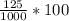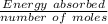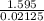Chemistry, 21.04.2020 22:43 yoyoho6218
You mix 125 mL of 0.170 M with 50.0 mL of 0.425 M in a coffee-cup calorimeter, and the temperature of both solutions rises from 20.20 °C before mixing to 22.17 °C after the reaction. What is the enthalpy of reaction per mole of ? Assume the densities of the solutions are all 1.00 g/mL, and the specific heat capacities of the solutions are 4.2 J/g · K. Enthalpy of reaction = kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
You mix 125 mL of 0.170 M with 50.0 mL of 0.425 M in a coffee-cup calorimeter, and the temperature o...
Questions

Mathematics, 03.08.2021 18:40

History, 03.08.2021 18:40






Physics, 03.08.2021 18:40


Health, 03.08.2021 18:40


Mathematics, 03.08.2021 18:40





Mathematics, 03.08.2021 18:40