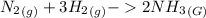Chemistry, 21.04.2020 20:17 amypeace1978
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L. The following reaction takes place: N2(g) 3 H2(g) 2 NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant. g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L...
Questions








Mathematics, 15.04.2020 17:24

Mathematics, 15.04.2020 17:24



Mathematics, 15.04.2020 17:24


Mathematics, 15.04.2020 17:24



English, 15.04.2020 17:24