Chemistry, 21.04.2020 15:24 jasminecoronetti44
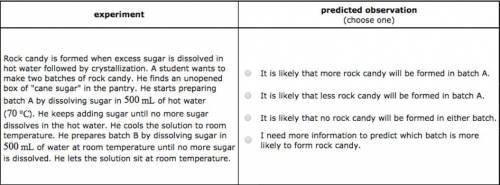
Rock candy is formed when excess sugar is dissolved in hot water followed by crystallization. A student wants to make two batches of rock candy. He finds an unopened box of "cane sugar" in the pantry. He starts preparing batch A by dissolving sugar in of hot water (). He keeps adding sugar until no more sugar dissolves in the hot water. He cools the solution to room temperature. He prepares batch B by dissolving sugar in of water at room temperature until no more sugar is dissolved. He lets the solution sit at room temperature. It is likely that less rock candy will be formed in batch A. It is likely that no rock candy will be formed in either batch. I need more information to predict which batch is more likely to form rock candy.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Rock candy is formed when excess sugar is dissolved in hot water followed by crystallization. A stud...
Questions

Chemistry, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Computers and Technology, 02.03.2021 19:10

Chemistry, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10







World Languages, 02.03.2021 19:10





Chemistry, 02.03.2021 19:10