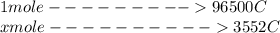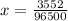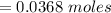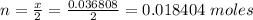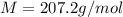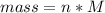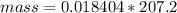Chemistry, 21.04.2020 15:30 sophcent5828
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate is reduced to lead at the cathode and oxidized to solid lead(II) oxide at the anode. Suppose a current of is fed into a car battery for seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

You know the right answer?
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentiall...
Questions

Mathematics, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00


Mathematics, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00




Biology, 04.08.2019 15:00

Spanish, 04.08.2019 15:00

Biology, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00


Biology, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00

History, 04.08.2019 15:00

Mathematics, 04.08.2019 15:00

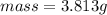
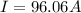
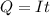
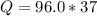
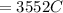
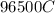
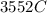 would contain how many moles
would contain how many moles