
Chemistry, 21.04.2020 15:29 hindjssnsk
A chemist must dilute 47.2 mL of 150. mM aqueous sodium nitrate solution until the concentration falls to . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

You know the right answer?
A chemist must dilute 47.2 mL of 150. mM aqueous sodium nitrate solution until the concentration fal...
Questions

Chemistry, 04.04.2020 09:24







Mathematics, 04.04.2020 09:25


Mathematics, 04.04.2020 09:25

Mathematics, 04.04.2020 09:25

Mathematics, 04.04.2020 09:25





Geography, 04.04.2020 09:26



Mathematics, 04.04.2020 09:26

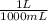 = 0.295 L
= 0.295 L

