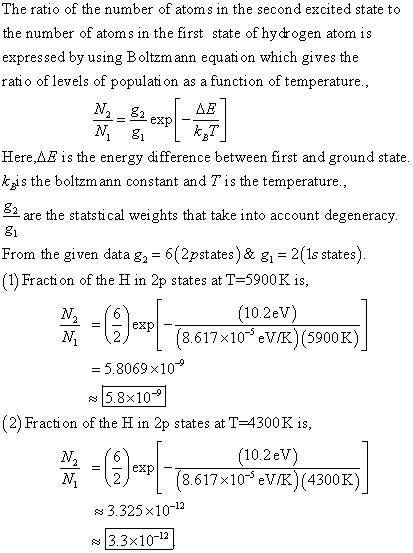
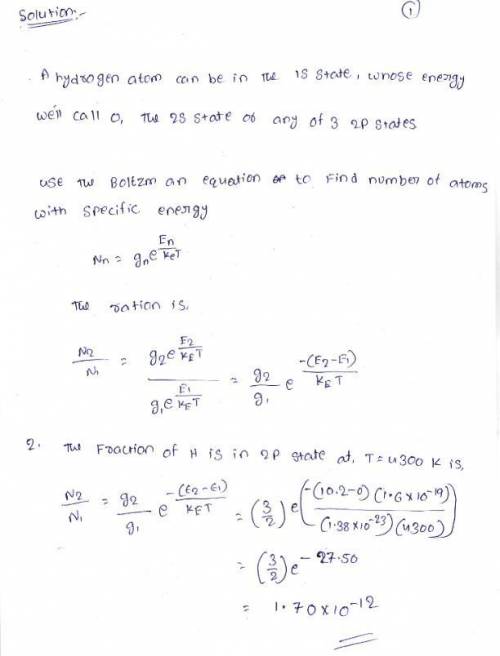
A hydrogen atom can be in the 1S state, whose energy we'll call 0, the 2S state, or any of 3 2P states. The 2S and 2P states have energies of 10.2 eV. There are other states with higher energy but we'll ignore them for simplicity. The 2P states have distinctive optical properties, so we're interested in how many are present even when it's a small fraction of the total?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1


Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1
You know the right answer?
A hydrogen atom can be in the 1S state, whose energy we'll call 0, the 2S state, or any of 3 2P stat...
Questions

Mathematics, 02.11.2019 06:31


Mathematics, 02.11.2019 06:31


Mathematics, 02.11.2019 06:31



History, 02.11.2019 06:31


English, 02.11.2019 06:31


Mathematics, 02.11.2019 06:31

Mathematics, 02.11.2019 06:31


English, 02.11.2019 06:31




Mathematics, 02.11.2019 06:31

History, 02.11.2019 06:31