
The following reaction was used to fuel the rockets in the Apollo mission landing module.
A) Is this reaction endothermic or exothermic?
B) How many grams of N2H4 must be reacted with an excess of N2O4 to produce 775 kJ of energy?
C) How many kJ of energy are produced when 6.25 g of N2O4 reacts with an excess of N2H4?
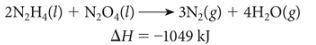

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
The following reaction was used to fuel the rockets in the Apollo mission landing module.
Questions


Advanced Placement (AP), 11.10.2019 03:30

Spanish, 11.10.2019 03:30

History, 11.10.2019 03:30

Mathematics, 11.10.2019 03:30

Social Studies, 11.10.2019 03:30

Biology, 11.10.2019 03:30

Computers and Technology, 11.10.2019 03:30


Chemistry, 11.10.2019 03:30


Mathematics, 11.10.2019 03:30




History, 11.10.2019 03:30

History, 11.10.2019 03:30

Physics, 11.10.2019 03:30





