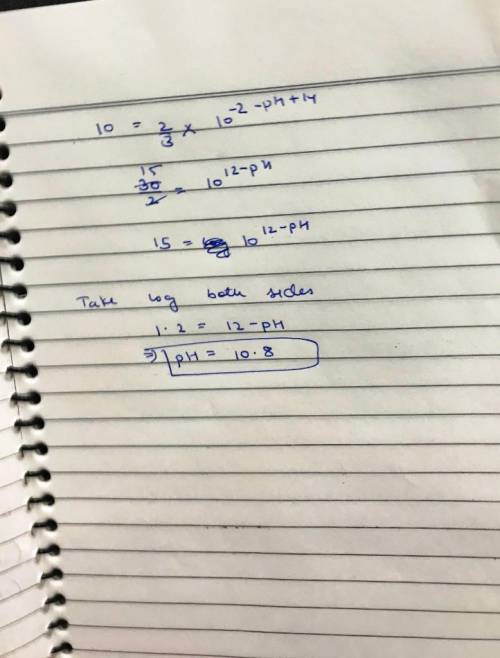Chemistry, 16.04.2020 00:17 sarahhN7534
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purification. The kinetics of such decomposition has been presented in Chemical Reviews, November 2001. There are two possible pathways for these reactions, one unimolecular and the other bimolecular with the help of OH- ions.
Path 1. BrCl2CCO2- + H2O goes to CHCl2Br + HCO3-
With a pseudo-first-order rate constant k1=1.6 x 10-6 1/sec
Path 2. BrCl2CCO2- + OH- goes to Cl2OHCCO2- + Br-
With a second-order rate constant k2=2.4x10-4 1/(M sec)
(a) Write the overall rate law for the disappearance of BrCl2CCO2.
(b) What is the ratio of the rates for paths 1 and 2 at pH=7?
(c) At what pH would the rate for path 2 be 10% of the rate for path 1?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


You know the right answer?
The decomposition of Bromodichloroacetate BrCl2CCO2- is an important required step in water purifica...
Questions



Mathematics, 29.08.2019 22:30

Chemistry, 29.08.2019 22:30

Mathematics, 29.08.2019 22:30




English, 29.08.2019 22:30

Chemistry, 29.08.2019 22:30

Computers and Technology, 29.08.2019 22:30




Computers and Technology, 29.08.2019 22:30

Biology, 29.08.2019 22:30


Engineering, 29.08.2019 22:30