Chemistry, 15.04.2020 03:54 Ashley606hernandez
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with oxygen according to the following equation (which may or may not be balanced): B2H6 (g) + O2 (g) → B2O3 (s) + H2O (l) How many grams of O2 (molar mass 32.00 g/mol) will react with 14.67 grams of diborane (molar mass 27.67 g/mol). Your answer must be expressed to the correct number of significant figures, and with the correct unit.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








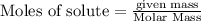
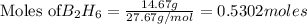
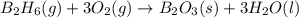
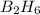 require = 3 moles of
require = 3 moles of 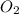
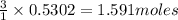 of
of