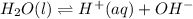Chemistry, 15.04.2020 03:33 ramjand7853
When a reaction system is at equilibrium: a. there is no more chemistry happening. b. the amounts of reactants and products are exactly equal. c. the reaction rate in the forward direction is at a maximum. d. the reaction rate in the reverse direction is at a minimum. e. the rates of the reaction in the forward and reverse directions are exactly equal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
When a reaction system is at equilibrium: a. there is no more chemistry happening. b. the amounts of...
Questions

Mathematics, 08.06.2021 17:40


Health, 08.06.2021 17:40


Biology, 08.06.2021 17:40

Mathematics, 08.06.2021 17:40

Mathematics, 08.06.2021 17:40



Mathematics, 08.06.2021 17:40


Mathematics, 08.06.2021 17:40




Mathematics, 08.06.2021 17:40




Biology, 08.06.2021 17:40

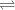 " in a chemical equation.
" in a chemical equation.