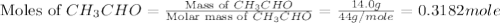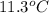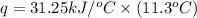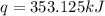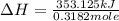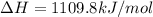Chemistry, 15.04.2020 03:33 tusharchandler124
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combustion = −803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.0-g sample of acetaldehyde (CH3CHO) produced a temperature increase of 11.3°C in the same calorimeter. What is the energy of combustion of acetaldehyde (in kJ/mol)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combu...
Questions

English, 21.07.2019 04:30

Mathematics, 21.07.2019 04:30


Biology, 21.07.2019 04:30



Health, 21.07.2019 04:30


History, 21.07.2019 04:30

Mathematics, 21.07.2019 04:30

Mathematics, 21.07.2019 04:30

Computers and Technology, 21.07.2019 04:30

Arts, 21.07.2019 04:30

Computers and Technology, 21.07.2019 04:30


Mathematics, 21.07.2019 04:30

Mathematics, 21.07.2019 04:30



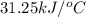
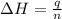
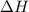 = enthalpy change = -803 kJ/mol
= enthalpy change = -803 kJ/mol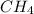 = 6.91 g
= 6.91 g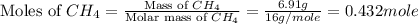
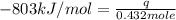
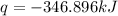
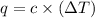
 = change in temperature =
= change in temperature = 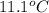
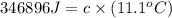
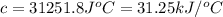
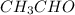 = 14.0 g
= 14.0 g