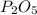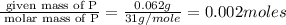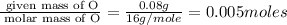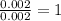Chemistry, 15.04.2020 03:03 frankieflores02
A form of phosphorus called red phosphorus is used in match heads. When 0.062 g of red phosphorus burns in air, it forms 0.142 g of phosphorus oxide. Determine the empirical formula of phosphorus oxide.(show work; use labels)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
A form of phosphorus called red phosphorus is used in match heads. When 0.062 g of red phosphorus bu...
Questions

English, 13.10.2020 18:01


Mathematics, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01



Advanced Placement (AP), 13.10.2020 19:01

Mathematics, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01




Mathematics, 13.10.2020 19:01



Mathematics, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01

Computers and Technology, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01