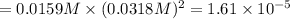PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)

Chemistry, 15.04.2020 00:52 genyjoannerubiera
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Suppose you add 0.2307 g of PbCl2 (s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of Pb2+ (aq) is 0.0159 M and the concentration of Cl− (aq) is 0.0318 M.
What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

You know the right answer?
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Questions





Mathematics, 25.06.2019 12:20










Mathematics, 25.06.2019 12:20





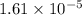 .
.![[Pb^{2+}]=0.0159 M](/tpl/images/0600/3825/89ec5.png)
![[Cl^-]=0.0318 M](/tpl/images/0600/3825/75929.png)
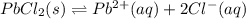
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0600/3825/7fd11.png)