Chemistry, 15.04.2020 02:09 claudia122752
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphite) + 2 H2(g) + O2(g) ΔH° = 232 kJ What is the standard enthalpy change for this reaction at 298 K? C(s, graphite) + H2(g) + 1/2 O2(g) H2CO(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

You know the right answer?
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphit...
Questions


History, 28.03.2021 23:50

Mathematics, 28.03.2021 23:50

Social Studies, 28.03.2021 23:50



Mathematics, 28.03.2021 23:50



Mathematics, 28.03.2021 23:50


History, 28.03.2021 23:50

Mathematics, 28.03.2021 23:50

Mathematics, 28.03.2021 23:50

Mathematics, 28.03.2021 23:50

English, 28.03.2021 23:50


Mathematics, 28.03.2021 23:50

Chemistry, 28.03.2021 23:50

Mathematics, 28.03.2021 23:50

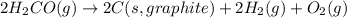
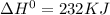
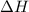 for the following reaction i.e,
for the following reaction i.e,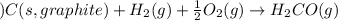
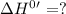
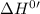 for the reaction will be:
for the reaction will be: