
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of gas A and gas B, respectively, are 0.419 atm 0.419 atm and 0.589 atm. 0.589 atm. If 0.240 mol 0.240 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
A 9.75 L 9.75 L container holds a mixture of two gases at 41 ° C. 41 °C. The partial pressures of ga...
Questions

Biology, 30.01.2020 01:42



Mathematics, 30.01.2020 01:42



Chemistry, 30.01.2020 01:42


History, 30.01.2020 01:42




Mathematics, 30.01.2020 01:42

Mathematics, 30.01.2020 01:42


Mathematics, 30.01.2020 01:42


Physics, 30.01.2020 01:42

Mathematics, 30.01.2020 01:42

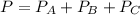 ----- (1)
----- (1) atm
atm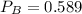 atm
atm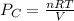

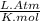
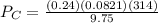
 = 0.634 atm
= 0.634 atm

