
Chemistry, 14.04.2020 18:05 rileyeddins1010
What is the % yield of the reaction making 1.6 g of acetyl salicylic acid from 5.7 g of salicylic acid, 9.9 mL of acetic anhydride (density 1.082), and 10 drops 85 % weight phosphoric acid (density 1.685)? Use the molecular weights that you have already calculated. Give two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
What is the % yield of the reaction making 1.6 g of acetyl salicylic acid from 5.7 g of salicylic ac...
Questions


Mathematics, 28.01.2021 01:00


History, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

English, 28.01.2021 01:00


Biology, 28.01.2021 01:00

Spanish, 28.01.2021 01:00


History, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00




Mathematics, 28.01.2021 01:00

Chemistry, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00


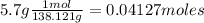
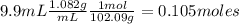
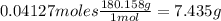
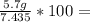 76.7%
76.7%

