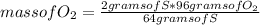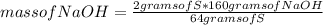Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Which substance is the limiting reactant when 2.0 g of sulfur reacts with 3.0 g of oxygen and 4.0 g...
Questions


History, 20.03.2020 17:02





Computers and Technology, 20.03.2020 17:04

Mathematics, 20.03.2020 17:04


Mathematics, 20.03.2020 17:04


History, 20.03.2020 17:04


Biology, 20.03.2020 17:04