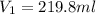Chemistry, 08.04.2020 04:33 damonsmith201615
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out some aqueous calcium sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the calcium sulfate stock solution that the chemist should pour out. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out som...
Questions

Mathematics, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

Chemistry, 05.05.2021 06:30



Health, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

Chemistry, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30



Mathematics, 05.05.2021 06:30

Chemistry, 05.05.2021 06:30

English, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

German, 05.05.2021 06:30

Mathematics, 05.05.2021 06:30

Computers and Technology, 05.05.2021 06:30

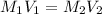
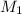 = molarity of stock calcium sulphate solution = 1.82 M
= molarity of stock calcium sulphate solution = 1.82 M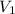 = volume of stock calcium sulphate solution = ?
= volume of stock calcium sulphate solution = ?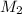 = molarity of dilute calcium sulphate solution = 1.00 M
= molarity of dilute calcium sulphate solution = 1.00 M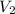 = volume of dilute calcium sulphate solution = 400 ml
= volume of dilute calcium sulphate solution = 400 ml