
The reaction of UO2 with hydrogen ion in aqueous solution 2 UO2 4 H U4 UO22 2 H2O is second order in UO2 and third order overall. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

You know the right answer?
The reaction of UO2 with hydrogen ion in aqueous solution 2 UO2 4 H U4 UO22 2 H2O is second order in...
Questions


Spanish, 09.07.2019 07:00

World Languages, 09.07.2019 07:00

World Languages, 09.07.2019 07:00

History, 09.07.2019 07:00

English, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00





Mathematics, 09.07.2019 07:00


History, 09.07.2019 07:00



Biology, 09.07.2019 07:00


History, 09.07.2019 07:00

![rate=k[UO_2^+]^2[H^+]](/tpl/images/0588/8942/4aa9a.png)
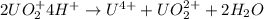
![rate=k[UO_2^+]^n[H^+]^m](/tpl/images/0588/8942/944b7.png)
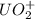 = 2
= 2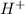 = ?
= ?![rate=k[UO_2^+]^2[H^+]^1](/tpl/images/0588/8942/77310.png)


