
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 mole glucose produces 0.5 mol glycerol and that the ratio of NH3 to H20 is 1:1. During preparation , you accidentally add too much glucose. However, you decide to let the bioreactor run anyway. After the reaction goes to completion at least one of the starting reactants is used up, you discover that 1.4 lb-m ethanol had been produced. How much heat had been produced and subsequently removed from the system. How much glucose did you initially add to the reactor?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 m...
Questions

Chemistry, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00

Medicine, 21.01.2021 20:00





Biology, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00

Mathematics, 21.01.2021 20:00

Mathematics, 21.01.2021 20:00

History, 21.01.2021 20:00



Mathematics, 21.01.2021 20:00

English, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00

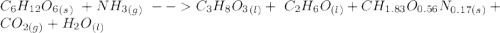
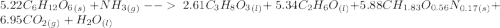
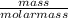
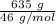
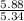 moles of S. cerevisiae formed.
moles of S. cerevisiae formed.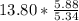 moles of S. cerevisiae formed
moles of S. cerevisiae formed

