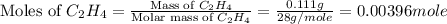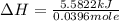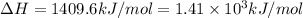Chemistry, 07.04.2020 15:12 kimberlylove387
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of ethylene (C 2H 4) was burned in this calorimeter, the temperature increased by 2.26 K. Calculate the energy of combustion for one mole of ethylene. a. -50.3 kJ/mol b. -1.41 x 103 kJ/mol c. -0.274 kJ/mol d. -624 kJ/mol e. -5.29 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
You know the right answer?
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of et...
Questions




Mathematics, 12.04.2021 21:30



Mathematics, 12.04.2021 21:30


Mathematics, 12.04.2021 21:30



Chemistry, 12.04.2021 21:30

Social Studies, 12.04.2021 21:30

Mathematics, 12.04.2021 21:30


Mathematics, 12.04.2021 21:30


Chemistry, 12.04.2021 21:30


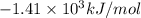
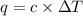
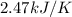
 = Change in temperature = 2.26 K
= Change in temperature = 2.26 K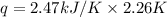
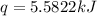
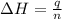
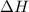 = energy of combustion for one mole of ethylene = ?
= energy of combustion for one mole of ethylene = ?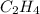 = 0.111 g
= 0.111 g