
Chemistry, 06.04.2020 01:02 winterblackburn78
According to the following equation, Calculate the percentage yield if 550.0 g of toluene (C7H8 )added to an excess of nitric acid (HNO3) provides 305 g of the p-nitrotoluene (C7H7NO2 ) product in a lab experiment.
___C7H8 + ___HNO3 ___C7H7NO2 + ___H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
According to the following equation, Calculate the percentage yield if 550.0 g of toluene (C7H8 )add...
Questions





Health, 12.06.2021 16:20


English, 12.06.2021 16:30

English, 12.06.2021 16:30

Mathematics, 12.06.2021 16:30



English, 12.06.2021 16:30


Mathematics, 12.06.2021 16:30

English, 12.06.2021 16:30

Mathematics, 12.06.2021 16:30

Mathematics, 12.06.2021 16:30

Social Studies, 12.06.2021 16:30


Mathematics, 12.06.2021 16:30

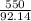
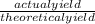 x 100
x 100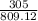 x 100
x 100

