
Chemistry, 12.06.2021 16:20 laiba012305
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide. 10.23 mL of the calcium hydroxide is required to reach the equivalence point. What is the molarity of the acetic acid in the vinegar.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2

Chemistry, 23.06.2019 10:50
Which compound should undergo substitution of the bromine by phenolate anion? draw the structure of the organic product?
Answers: 1
You know the right answer?
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide....
Questions

Mathematics, 29.01.2020 05:57




History, 29.01.2020 05:57


Biology, 29.01.2020 05:57


Biology, 29.01.2020 05:57





Chemistry, 29.01.2020 05:57



History, 29.01.2020 05:57

Chemistry, 29.01.2020 05:57


History, 29.01.2020 05:57

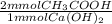 = 15.46 mmol CH₃COOH
= 15.46 mmol CH₃COOH

