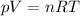What is the molar mass of a gas with a density of 3.50 g/L at a
temperature of 345 K and a pre...

Chemistry, 05.04.2020 22:22 raymondanthony3314
What is the molar mass of a gas with a density of 3.50 g/L at a
temperature of 345 K and a pressure of 0.950 atm? (Write your
answer whole number e. g. 345)
g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2


Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Questions

English, 15.12.2020 03:10


World Languages, 15.12.2020 03:10

English, 15.12.2020 03:10


Mathematics, 15.12.2020 03:10






Social Studies, 15.12.2020 03:10

Social Studies, 15.12.2020 03:10

History, 15.12.2020 03:10

Chemistry, 15.12.2020 03:10


Mathematics, 15.12.2020 03:10


Mathematics, 15.12.2020 03:10

English, 15.12.2020 03:10