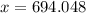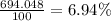Chemistry, 04.04.2020 11:42 PrincessIndia
Lithium has two stable isotopes with masses of 6.01512 amu and 7.01600 amu. The average molar mass of Li is 6.941 amu. What is the percent abundance of each isotope? Show all calculations and report to the correct number of sig figs.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3


Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

You know the right answer?
Lithium has two stable isotopes with masses of 6.01512 amu and 7.01600 amu. The average molar mass o...
Questions

English, 26.11.2019 07:31

Health, 26.11.2019 07:31

Mathematics, 26.11.2019 07:31

Health, 26.11.2019 07:31


History, 26.11.2019 07:31



Mathematics, 26.11.2019 07:31




English, 26.11.2019 07:31



Mathematics, 26.11.2019 07:31

History, 26.11.2019 07:31



Geography, 26.11.2019 07:31

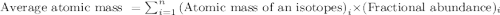 .....(1)
.....(1)
![6.941=[(6.01512\times x)+(7.01600\times (100-x))]](/tpl/images/0582/2997/75f4c.png)