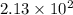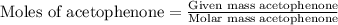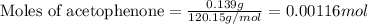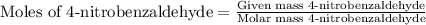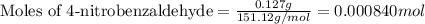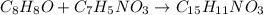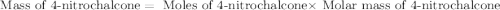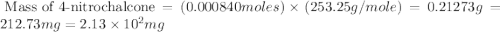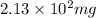Chemistry, 04.04.2020 11:45 juliopejfuhrf5447
According to the experimental procedure of Experiment F1, 135 microliters of acetophenone (120.15 g/mol, 1.03 g/mL) was reacted with 127 mg of 4-nitrobenzaldehyde (151.12 g/mol). What is the theoretical yield, in milligrams (mg), of trans-4-nitrochalcone (253.25 g/mol)? Enter your answer as digits only (no units), using the proper number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
According to the experimental procedure of Experiment F1, 135 microliters of acetophenone (120.15 g/...
Questions

Mathematics, 14.12.2021 19:40

Mathematics, 14.12.2021 19:40

Mathematics, 14.12.2021 19:40

Mathematics, 14.12.2021 19:40

English, 14.12.2021 19:40


Mathematics, 14.12.2021 19:40

Chemistry, 14.12.2021 19:40

SAT, 14.12.2021 19:40


English, 14.12.2021 19:40


Mathematics, 14.12.2021 19:40

Biology, 14.12.2021 19:40

Biology, 14.12.2021 19:40

Mathematics, 14.12.2021 19:40


Social Studies, 14.12.2021 19:40


Chemistry, 14.12.2021 19:40