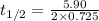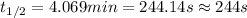Suppose the surface-catalyzed hydrogenation reaction of an unsaturated hydrocarbon has a rate constant of 0.725 M/min. The reaction is observed to follow zero-order kinetics. If the initial concentration of the hydrocarbon is 5.90 M, what is the half-life of the reaction in seconds? *Please report 3 significant figures. Numbers only, no unit. No scientific notation.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Suppose the surface-catalyzed hydrogenation reaction of an unsaturated hydrocarbon has a rate consta...
Questions
















Social Studies, 17.12.2019 00:31



Computers and Technology, 17.12.2019 00:31

![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0582/1472/b5b11.png)
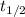 = half-life of the reaction = ?
= half-life of the reaction = ?![[A_o]](/tpl/images/0582/1472/dc622.png) = initial concentration = 5.90 M
= initial concentration = 5.90 M