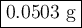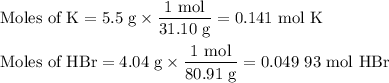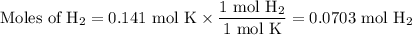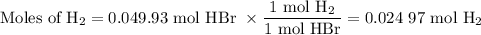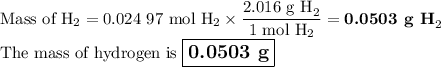Chemistry, 03.04.2020 06:24 spdesch2558
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
●C.)How much product is produced?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

You know the right answer?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...
Questions



English, 28.04.2021 19:30

Social Studies, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30



History, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30


Mathematics, 28.04.2021 19:30

Health, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30




Physics, 28.04.2021 19:30


Social Studies, 28.04.2021 19:30