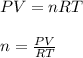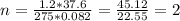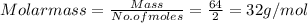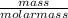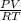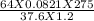Chemistry, 02.04.2020 02:43 PastyMexican24
A sample of gas has a mass of 64 g. Its volume is 37.6 L at a temperature of 275k and a pressure of 1.2 atm. Find its molar mass.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1


Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
A sample of gas has a mass of 64 g. Its volume is 37.6 L at a temperature of 275k and a pressure of...
Questions


English, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20




Mathematics, 15.12.2020 19:20


Mathematics, 15.12.2020 19:20

History, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20


Mathematics, 15.12.2020 19:20

Mathematics, 15.12.2020 19:20



Mathematics, 15.12.2020 19:20

English, 15.12.2020 19:20


Biology, 15.12.2020 19:20