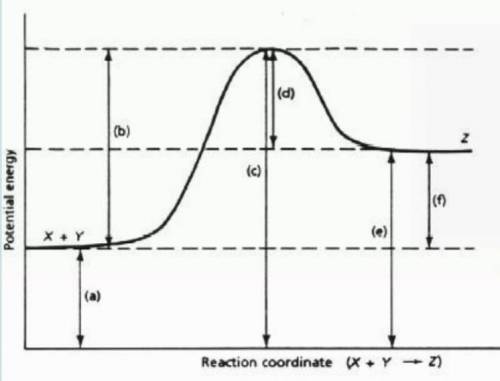
Which letter (a-f) represents activation energy (Ea) of the forward reaction (reactants)?
Whic...

Chemistry, 02.04.2020 02:43 tinktkinkdavis7340
Which letter (a-f) represents activation energy (Ea) of the forward reaction (reactants)?
Which letter (a-f) represents activation energy (Ea) of the reverse reaction (products)?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Questions

Arts, 12.04.2021 17:00

Computers and Technology, 12.04.2021 17:00

English, 12.04.2021 17:00

Mathematics, 12.04.2021 17:00

Mathematics, 12.04.2021 17:00

English, 12.04.2021 17:00

Geography, 12.04.2021 17:00


Mathematics, 12.04.2021 17:00


Biology, 12.04.2021 17:00




English, 12.04.2021 17:00


Mathematics, 12.04.2021 17:00

History, 12.04.2021 17:00

Mathematics, 12.04.2021 17:00

English, 12.04.2021 17:00



