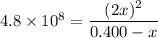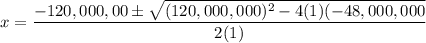H2(g) + Br2(l) ⇄ 2HBr(g) Kc = 4.8 × 108
Assume initial conditions of 0.400 M H2(g) and e...

Chemistry, 01.04.2020 20:16 CHRONICxDJ
H2(g) + Br2(l) ⇄ 2HBr(g) Kc = 4.8 × 108
Assume initial conditions of 0.400 M H2(g) and excess Br2(l). What is the equilibrium concentration of H2(g)?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Questions

History, 03.05.2021 19:20


Mathematics, 03.05.2021 19:20


Mathematics, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20



Mathematics, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20

World Languages, 03.05.2021 19:20

Chemistry, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20



Chemistry, 03.05.2021 19:20

Chemistry, 03.05.2021 19:20

History, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20

Mathematics, 03.05.2021 19:20

![k_c=\dfrac{[HBr(g)]^2}{[H_2]}=4.8\times 10^8M](/tpl/images/0576/0517/a3b30.png)