
Chemistry, 03.05.2021 19:20 emmmmmmaaaa
For this reaction: 6 PbO + O2 → 2 Pb304 a. How many moles of Pb304 are produced from 1.25 moles of oxygen?
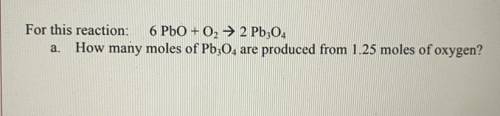

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
For this reaction: 6 PbO + O2 → 2 Pb304
a. How many moles of Pb304 are produced from 1.25 moles of...
Questions

Chemistry, 29.10.2020 17:00






Computers and Technology, 29.10.2020 17:00


Computers and Technology, 29.10.2020 17:00



Business, 29.10.2020 17:00





Mathematics, 29.10.2020 17:00





