
Chemistry, 31.03.2020 14:40 ashley232323
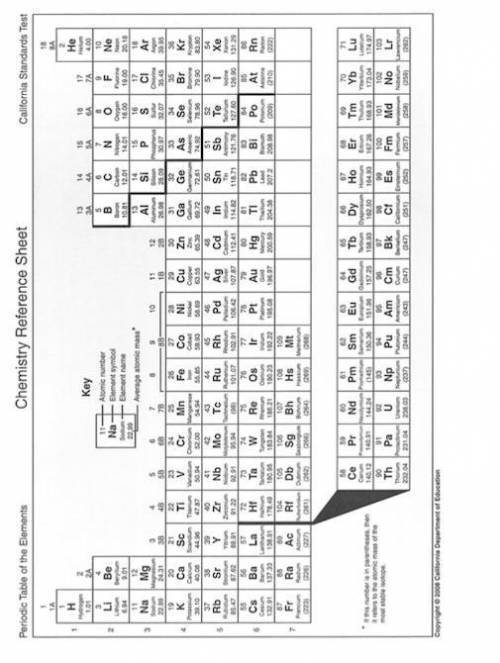
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Periodic Table on page 2 to calculate the molar masses.
(i) A solution is prepared using 17 g of water and 1 g of NaNO3. What is the molality of the NaNO3 solution?
(ii) In order to prepare a solution of 2 molal NaNO3 using 30 g of water,
what mass of NaNO3 is required?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2



Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Solve the below problems relating to the molality of NaNO3 in aqueous solutions. Make use of the Per...
Questions

Mathematics, 16.10.2020 02:01

Geography, 16.10.2020 02:01

English, 16.10.2020 02:01






Mathematics, 16.10.2020 02:01

Mathematics, 16.10.2020 02:01



Chemistry, 16.10.2020 02:01

Business, 16.10.2020 02:01


Mathematics, 16.10.2020 02:01



Mathematics, 16.10.2020 02:01




