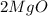Chemistry, 31.03.2020 14:41 teesoprettyy
12g of magnesium ribbon was heated in a crucible. At the end of the reaction 20g of magnesium oxide had been produced. What mass of oxygen had reacted with the magnesium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
12g of magnesium ribbon was heated in a crucible. At the end of the reaction 20g of magnesium oxide...
Questions



Mathematics, 29.10.2020 03:10





Mathematics, 29.10.2020 03:10



Mathematics, 29.10.2020 03:10


History, 29.10.2020 03:10


English, 29.10.2020 03:10


Mathematics, 29.10.2020 03:10


Physics, 29.10.2020 03:10

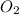 reacted with the magnesium
reacted with the magnesium