

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

You know the right answer?
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481...
Questions











Mathematics, 13.04.2021 04:40





Mathematics, 13.04.2021 04:40




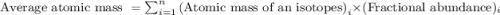 .....(1)
.....(1)![\text{Average atomic mass of element}=[(281.99481\times 0.60795)+(283.99570\times 0.22122)+(286.99423\times 0.17083)]\\\\\text{Average atomic mass of element}=283.291amu](/tpl/images/0572/8936/1f7da.png)


