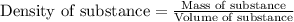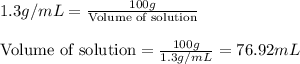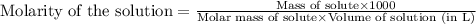Chemistry, 31.03.2020 01:50 jazminjb83
We have an aqueous solution that contains 23% (by mass) of a hypothetical solute Z. The formula weight of the solute Z is 139 g/mol. The density of the solution is observed to be 1.3 g/mL. What is the molarity of Z in this solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
We have an aqueous solution that contains 23% (by mass) of a hypothetical solute Z. The formula weig...
Questions

History, 01.02.2021 06:50





Mathematics, 01.02.2021 06:50


Mathematics, 01.02.2021 06:50



English, 01.02.2021 06:50

Mathematics, 01.02.2021 06:50



English, 01.02.2021 06:50

Social Studies, 01.02.2021 06:50


Mathematics, 01.02.2021 06:50

Biology, 01.02.2021 06:50