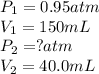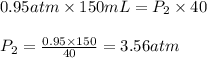Chemistry, 31.03.2020 01:30 mjstew00763
A sample of helium gas has a volume of 150. Ml (V₁) at 0.95 atom (P₁). What will your new pressure (P₁) be if the volume is changed to 40.0 ml (V₁) and temperature remains constant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
A sample of helium gas has a volume of 150. Ml (V₁) at 0.95 atom (P₁). What will your new pressure (...
Questions

Mathematics, 01.04.2020 01:32




Mathematics, 01.04.2020 01:32

Mathematics, 01.04.2020 01:32

Mathematics, 01.04.2020 01:32

Social Studies, 01.04.2020 01:32

Biology, 01.04.2020 01:32


Social Studies, 01.04.2020 01:32



Physics, 01.04.2020 01:32

Mathematics, 01.04.2020 01:32



Biology, 01.04.2020 01:32

History, 01.04.2020 01:32

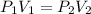
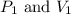 are initial pressure and volume.
are initial pressure and volume.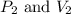 are final pressure and volume.
are final pressure and volume.