
Chemistry, 31.03.2020 00:57 jettskii214
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylamine, CH3NH2(aq), with 0.200 M HCl(aq): (a) before addition of any HCl(aq) (b) after addition of 17.5 mL of HCl(aq) (c) after addition of 34.9 mL of HCl(aq) (d) after addition of 35.0 mL of HCl(aq) (e) after addition of 35.1 mL of HCl(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylami...
Questions


Mathematics, 21.03.2020 02:57


Computers and Technology, 21.03.2020 02:57

Mathematics, 21.03.2020 02:57





Mathematics, 21.03.2020 02:58

Mathematics, 21.03.2020 02:58





Law, 21.03.2020 02:58



Arts, 21.03.2020 02:58

Advanced Placement (AP), 21.03.2020 02:58

![K_{b} =\frac{[CH_{3}NH_{3}]+ [OH-] }{[CH_{3}NH_{2} ] }\\4.6x10^{-4} =\frac{x*x}{0.2-x} \\x=0.00936 M](/tpl/images/0572/2081/59e94.png)
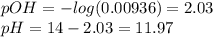
![[acid]=\frac{M_{2}V_{2} }{V_{1}+V_{2} } =\frac{0.2*17.5}{35+17.5} =0.0667M\\[base]=\frac{M_{1}V_{1} }{V_{1}+V_{2} } =\frac{0.2*35}{35+17.5} =0.13M](/tpl/images/0572/2081/75f14.png)
![pOH=pK_{b} +log(\frac{[salt]}{[base]} )=3.34+log(0.0667/0.066)=3.34\\pH=14-3.34=10.66](/tpl/images/0572/2081/0a576.png)
![[acid]=\frac{0.2*34.9}{35+34.9} =0.0998M\\\\ [base]=\frac{0.2*35}{35+34.9} =0.1M\\base-left=0.1-0.0998=0.0002M\\pOH=3.34+log(0.0998/0.0002)=6.04\\pH=14-6.04=7.96](/tpl/images/0572/2081/d31c6.png)
![[acid]=\frac{0.2*35}{35+35} =0.1M\\[base]=\frac{0.2*35}{35+35} =0.1M\\\\pH=7-\frac{1}{2} (pK_{b}+ log(c))=7-\frac{1}{2}(3.34+log(0.1))=5.83 \\](/tpl/images/0572/2081/b3303.png)
![[acid]=\frac{0.2*35.1}{35+35.1} =0.1M\\[base]=\frac{0.2*35}{35+35.1} =0.0998M\\acid-left=0.1-0.0998=0.0002M\\pH=-log(0.0002)=3.7\\](/tpl/images/0572/2081/1cd67.png)


