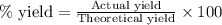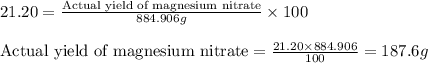Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Assuming an efficiency of 21.20 % , calculate the actual yield of magnesium nitrate formed from 143....
Questions


Mathematics, 20.06.2021 02:20




Mathematics, 20.06.2021 02:20

Mathematics, 20.06.2021 02:20


Chemistry, 20.06.2021 02:20

Mathematics, 20.06.2021 02:20


English, 20.06.2021 02:20


English, 20.06.2021 02:20


Mathematics, 20.06.2021 02:20

Biology, 20.06.2021 02:20

Mathematics, 20.06.2021 02:20


Mathematics, 20.06.2021 02:30

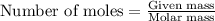 .....(1)
.....(1)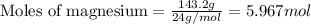
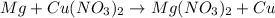
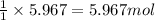 of magnesium nitrate
of magnesium nitrate