

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
If the equilibrium constant for the reaction 3I- (aq) + S2O82- (aq) I3- (aq) + 2SO42- (aq) is K, w...
Questions





History, 25.07.2019 15:40

Mathematics, 25.07.2019 15:40




Biology, 25.07.2019 15:40


Computers and Technology, 25.07.2019 15:40


Mathematics, 25.07.2019 15:40

Mathematics, 25.07.2019 15:40


Mathematics, 25.07.2019 15:40


History, 25.07.2019 15:40

![K'=\sqrt[3]{\frac{1}{K}}](/tpl/images/0572/2471/9553b.png)
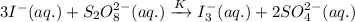 ......(1)
......(1)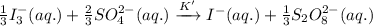 .........(2)
.........(2)

