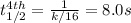Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
The reaction 3NO ---> N2O + NO2 is found to obey the rate law Rate = k[NO]^2^. If the first half-...
Questions



Chemistry, 04.12.2020 08:20

Chemistry, 04.12.2020 08:20


Social Studies, 04.12.2020 08:20


Health, 04.12.2020 08:20

Biology, 04.12.2020 08:20


Mathematics, 04.12.2020 08:20


Social Studies, 04.12.2020 08:20


Biology, 04.12.2020 08:20


English, 04.12.2020 08:20

Mathematics, 04.12.2020 08:20

Chemistry, 04.12.2020 08:20

Chemistry, 04.12.2020 08:20

![k=\frac{1}{[NO]_0*t_{/2}} = \frac{1}{[NO]_0*2.0}=\frac{1}{2[NO]_0}](/tpl/images/0572/1734/0a0ea.png)