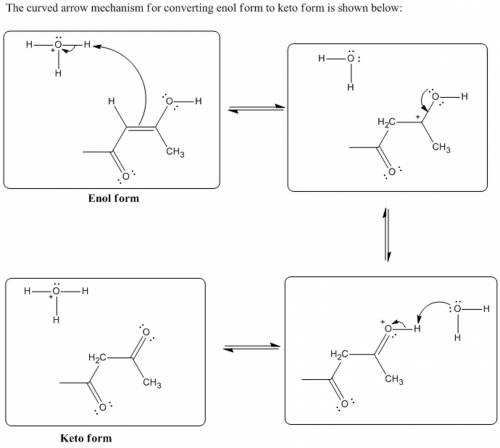
Chemistry, 31.03.2020 00:49 decemberbooker2505
Complete the mechanism for the keto-enol tautomerization below using bonds, charges, nonbonding electron pairs, and curved arrows (forward reaction only). Use the existing electrons and charges as guides to completing the mechanism of the tautomerization. Do not remove any pre-drawn bonds/lone pairs.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

You know the right answer?
Complete the mechanism for the keto-enol tautomerization below using bonds, charges, nonbonding elec...
Questions


Mathematics, 18.02.2020 20:13

Mathematics, 18.02.2020 20:13

History, 18.02.2020 20:13





Biology, 18.02.2020 20:13

Mathematics, 18.02.2020 20:13

Spanish, 18.02.2020 20:13






Chemistry, 18.02.2020 20:14


Mathematics, 18.02.2020 20:14