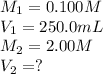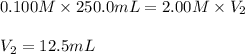Chemistry, 31.03.2020 00:27 shaymabejja1965
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solution of 2.00 M copper (II) chloride is available. How much of the stock solution is needed

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solut...
Questions





Advanced Placement (AP), 16.12.2020 16:30

History, 16.12.2020 16:30

Mathematics, 16.12.2020 16:30





Biology, 16.12.2020 16:30








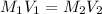
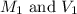 are the initial molarity and volume of copper (II) chloride.
are the initial molarity and volume of copper (II) chloride.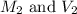 are the final molarity and volume of stock solution of copper (II) chloride.
are the final molarity and volume of stock solution of copper (II) chloride.