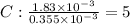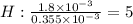Chemistry, 31.03.2020 00:27 CatsandDogsaredabest
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combustion of 29.5 mg produced 80.1 mg of CO2 and 16.4 mg of H2O. The molar mass of the compound was 162 g/mol. Determine its empirical and molecular formulas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combu...
Questions


English, 31.10.2019 06:31

Biology, 31.10.2019 06:31


Physics, 31.10.2019 06:31



Social Studies, 31.10.2019 06:31


History, 31.10.2019 06:31

Health, 31.10.2019 06:31

English, 31.10.2019 06:31


Mathematics, 31.10.2019 06:31


English, 31.10.2019 06:31

History, 31.10.2019 06:31

Biology, 31.10.2019 06:31


Mathematics, 31.10.2019 06:31

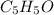 .
.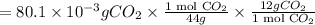
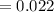 g
g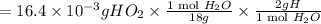
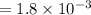 g
g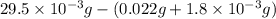
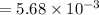 g
g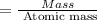
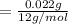
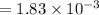 mol
mol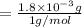
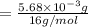
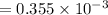 mol
mol